professional knowledge for practice
Services
Expert and efficiently networked – IGR services
Legal requirements are in a state of constant change, growing increasingly complex. Companies in the chemical and pharmaceutical industries must keep a host of technical codes and standards, regulations and laws in view. On behalf of them and their services providers, IGR brings together experts and decision-makers from planning, manufacturing and engineering.
IGR provides a knowledge basis, enabling its member companies to efficiently fulfil their technical compliance, i.e. their responsibilities as operators. This applies to the design, approval, installation, operation and maintenance of process-engineering and energy plants.
To this end, over 350 active experts from over 30 member companies regularly exchange ideas and experience, keeping track of the state of the art and applying tools to harness current developments for plant operators.
Services
Services
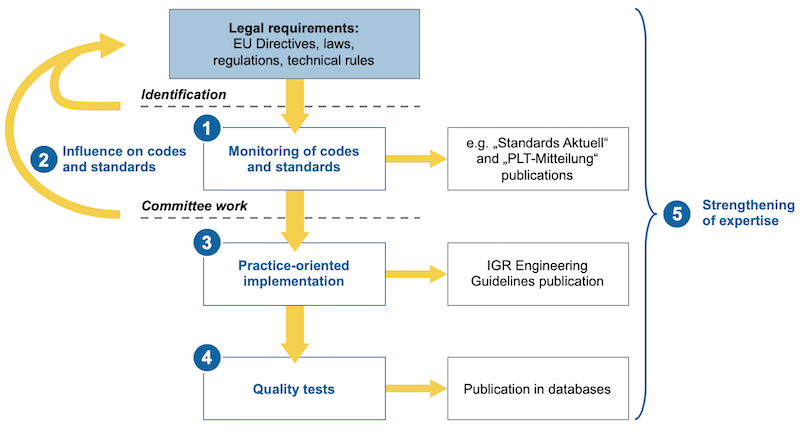
1. Overview & analysis
- Systematic identification and monitoring of regulations, codes and standards
2. Coordination & representation of interests
- Communication and exchange of experience and ideas between member companies and external service providers as well as authorised inspection agencies (AIAs)
- Summarising of laws, regulations and technological codes and standards for practice-focused implementation
- Participation in national and international committees
- Pooling of the interests of IGR members and organisation of their representation in external committees
3. Tools & contact partners
- Preparation of practice-focused instructions / guidelines and tools (e.g. Engineering Guidelines)
- Specifications for E/I&C equipment, process-engineering equipment and pipe components
- Regular publications, such as the “Standards Aktuell” and “IGR-Aktuell” information newsletters (6 and 4 times per year respectively)
- Telephone contact with experienced experts
4. Standards & monitoring
- Enabling compliance with quality standards
- Validation of materials, E/I&C field devices and selected test methods
5. Advanced education & exchanges of experience
- Regular expert discussions, seminars, training, workshops
- IGR exchanges of experience every 2 years